
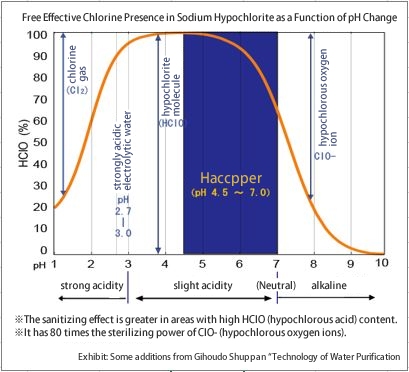
Haccpper water contains a large amount of hypochlorous acid (HOCl), which is formed when sodium hypochlorite (NaClO) reacts with water (H2O). The higher the content, the greater the sterilizing power.
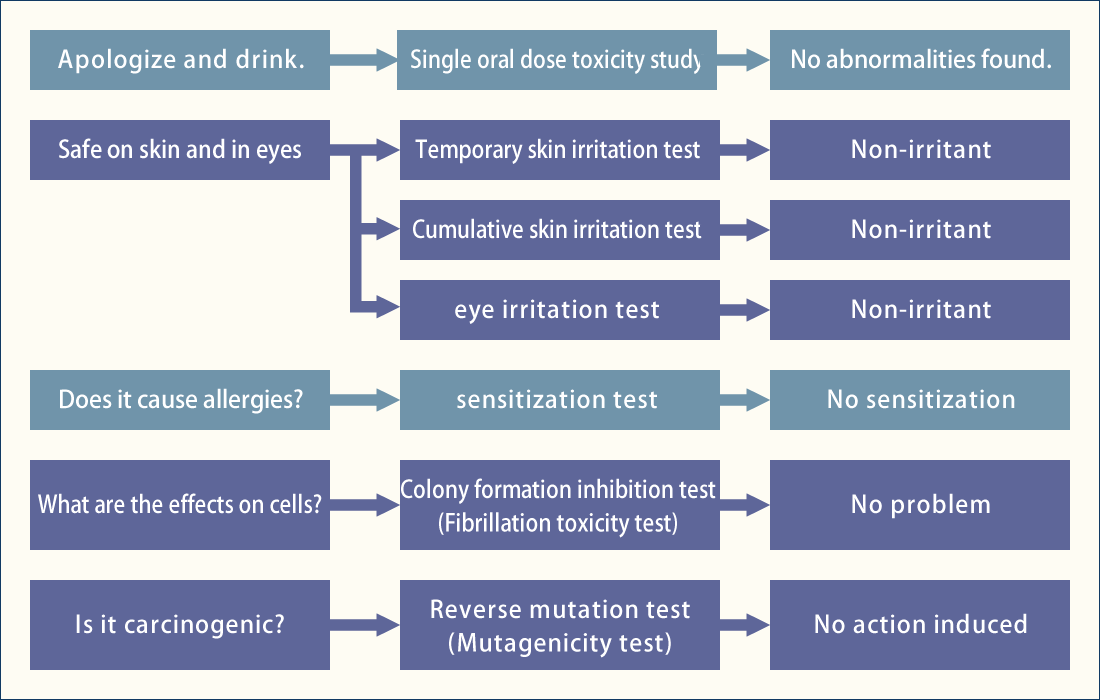
Haccpper Water is not new in that it uses sodium hypochlorite, but the difference is that by adjusting the pH of the sodium hypochlorite, a large amount of hypochlorous acid (HOCl), which is a bactericidal component contained in the sodium hypochlorite, is generated and used. There is a marked difference.
Sodium hypochlorite is widely used as a sanitizer in various situations such as food hygiene and environmental hygiene, etc. However, the Ministry of Health, Labor and Welfare (MHLW) has instructed that when it directly affects the human body, such as food, it should be diluted to 200ppm from a 12% concentration stock solution.
When sodium hypochlorite is diluted to 200 ppm, it becomes alkaline with a pH of 8.2 to 8.8. In this pH range, hypochlorous acid (HOCl), the active bactericidal ingredient, is present in only a little more than 10% of the solution. When the pH is reduced to a slightly acidic level below pH 7.0, the ratio increases dramatically to 70-100%, thereby strengthening the sterilizing power.
Even sodium hypochlorite with a pH of 8.2 to 8.8 can be diluted with water to bring it closer to pH 7. (Water is normally pH 7, so adding a large amount of water will bring it as close as possible to the same pH as water.) However, this method is not suitable for practical use because the concentration will be too thin and the sterilizing power will be too weak.
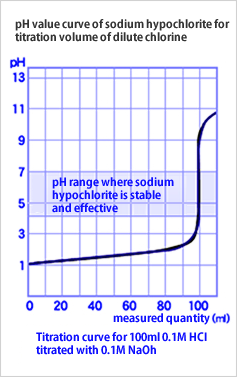
Haccpper mixes a low concentration of dilute hydrochloric acid (8.5%) with a very small amount of sodium hypochlorite to create water with a pH of 6.0 to 7.3, which contains a large amount of hypochlorous acid (HOCl), the effective disinfectant component. (See graph).
As you can see in the graph, the sterilizing power of Haccpper water increases even more when the pH of the water is around 5.
However, if the pH is too low (i.e., the acidity is too high), harmful chlorine gas (Cl2) is likely to be generated. For this reason, Haccpper is normally used at a pH of 5 or higher, a slightly acidic condition that does not generate chlorine gas.
Haccpper water cannot be put into practical use without technology to accurately control pH.
Haccpper water (hypochlorous acid) is produced by adding acid to sodium hypochlorite to control the pH value, but it has been considered difficult to automatically control sodium hypochlorite within a stable and effective pH range, and its practical application has been considered impossible.
Artificial mixing is very difficult and risky because a slight deviation in titration volume can quickly cause the pH value to go from below pH 3 to above pH 8, and chlorine gas can be generated if the pH value drops below pH 4.5.
Techno Max Co., Ltd. has established a technology to automate and accurately control these factors, and has succeeded in commercializing it.
Low concentration reduces damage and speeds up sterilization.
Can be safely sprayed in a normal working environment.
Instantaneous reaction due to chemical reaction.
The Haccpper system is a device that can scientifically synthesize and stably generate a large amount of sanitizing water containing a large amount of hypochlorous acid (HOCl).
The sanitizing water produced by this device is called Haccpper water.
The Haccpper System produces Haccpper water by diluting and mixing a large amount of water with sodium hypochlorite, a food additive, and 8.5% dilute hydrochloric acid, a food additive approved as an alkali neutralizing agent.
Haccpper water does not adhere to or remain on food products because it returns to normal water after coming into contact with bacteria and organic matter during the sterilization process. Even if there is some residual, it does not stick to the food like sodium hypochlorite, so it can be removed with a simple rinse.
In addition, compared to sodium hypochlorite, the concentration of hypochlorite is much lower and the sterilization time is shorter, so the taste and texture of the food will not be significantly impaired. Even if there is some residual hypochlorous acid in the wastewater, it will react with organic matter in the drainage ditch and return to water, so it will not damage the useful microorganisms in the septic tank.
The chemical formula for this is as follows

When Haccpper water is sprayed, it becomes a mist, and if it does not come in contact with organic matter such as bacteria in the air, the contained hypochlorous acid (HClO) decomposes and chlorine gas (Cl2) is generated.
HClO → H2O + O2 + Cl2
However, since chlorine gas ( Cl2 ) is very easily dissolved in water and dissolves into the sprayed water particles, the room is not filled with chlorine gas and the human body is not endangered.
| Effective chlorine concentration | 30 minutes later | 60 minutes later |
|---|---|---|
| pH5.89 500ppm | 0.10ppm | 0.12ppm * |
| pH5.82 200ppm | 0.02ppm | 0.07ppm * |
| pH5.95 100ppm | 0.01ppm以下* | 0.01ppm * |
| pH5.82 50ppm | 0.01ppm以下* | 0.01ppm以下* |
← 表は左右にスクロールできます →
* Figures in parentheses are actual measured values in a 20 m3 room with 4 L/hour spraying and no ventilation.
Experimental conditions: Room volume: 20 m3 (equivalent to about 6 tatami mats) (no ventilation during spraying), spray volume: 4 L/hour (floor is wet and needs to be wiped up with a mop.
Allowable concentration of chlorine gas, recommended by the Japan Society for Occupational Health: 0.5ppm.
It reacts instantaneously with the putrid odor of ammonia and chemically reacts to odorless monochloramine.
Sodium hypochlorite (NaClO) is most commonly used for deodorization (deodorization).
This is because sodium hypochlorite's oxidizing power is used to oxidize the source of the odor and convert it into an odorless substance. In the process of eliminating ammonia odor, the oxidizing power of sodium hypochlorite changes malodorous ammonia molecules into odorless monochloramine.
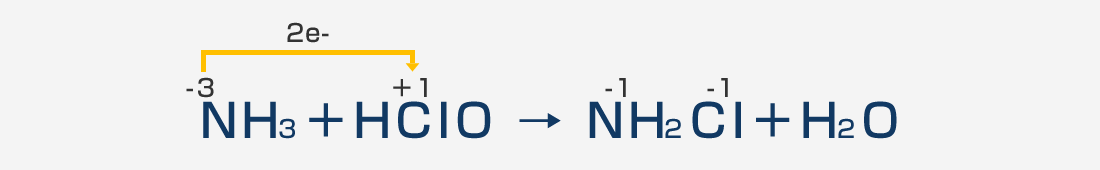
Haccpper water (HClO) has a higher oxidizing power than sodium hypochlorite, so its deodorizing effect is several to several dozen times higher as well as its sterilizing power, and can demonstrate a satisfactory deodorizing effect at a lower concentration.
Copyright © Techno Max Co.,Ltd. All Rights Reserved.